Recycling Graphite for Vanadium Redox Flow Batteries: Options for Usage and Critical Integredients | Thorsten Hickmann
Thorsten Hickmann | Recycling Graphite for Vanadium Redox Flow Batteries: Options for Usage and Critical Integredients
Mini Review
Abstract
Modern energy systems, such as redox flow batteries (RFB), recently are becoming increasingly popular in the special isttrade [1-5]. Despite a numberof demonstrative tests on redox flow batteries [4-7], upto now there does only orginal Carbon material exist. However, it is necessary to look at Recycling mateirals. There are a certain number how to get recycled graphite. However, when deciding to use these materials, it is necessary to have a close look of the characteristics of these materials.
Keywords: Recycling; Vanadium redox flow batteries; Graphite; Compound; Bipolar plates; Carbon; Recycling
Abbreviations: C: Carbon; FE: Iron; N: Nitrogen, SEM: Scanning Electrone Microscope; VRB: Vanadium Redox Flow Batteries
Introduction
Already in 2010, a working group of the European Commission, analysed arround 41 materials and came to the conclusion about their criticality and the risk of supply safety and their ecomic importance. As a result, around 14 matrials were judged critically, and this included graphite as well [8]. For some industry areas, Graphite is estimated as a critical material [9]. For this reason, the importance of Recycling Graphite is growing and it is necessary to look for recycling material.
Discussion
As for the option how to get the recycled graphite-material, there are different possibilities. These possibilities aresummarized in Table 1.
Table 1 shows different option show Recycling Carbon can be used. The table shows that there are 2 materials which have a good availability. Industrial Recycling Carbin and Recyclung out of Production. Since these condpathis evident how the material are composed together, it make sense to look at the material whis is from Industrial Recycling Graphite. For this reason, A Scanning Electoning Microscope (SEM) isused. With the SEM it is not possible to have a quantitative Anaylsys.
Table 1: Options of recycling carbon.
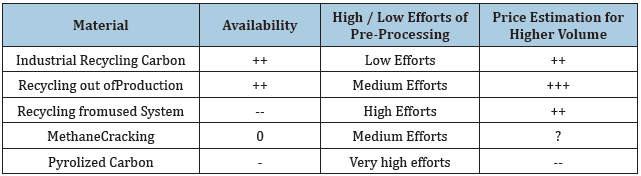
Figure 1 shows a SEM of a Carbon Composite material with 72 % Fillerof Industrial Recycling Carbon.
Figure 1: SEM of a carbon composite material filled with 72% graphite.
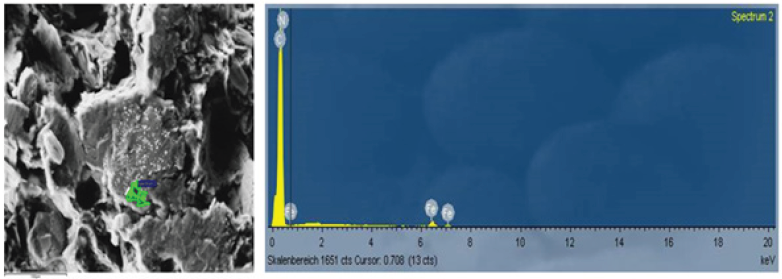
Looking at the SEM of Figure 1, the specrte of points is shown. The area with the green color: Here the iron-concentration is fairly high. In fact, Iron is contaminating the Redox Flow Battery, for this reason, it has to be avoided [7].
Thorsten Hickmann Articles from Crimson Publishers
Generally spoken, at a SEM the area of analysis is fairly small. During the test, Eisenhuth has taken 10 different test areas. However, it is possible the at there are still some other materials in the Compound. During, the measurement, there were found some iron and ironoxide particles. In addtion, the following materials could be found:
a) Calcium
b) Aluminium
c) Zirconium
d) Vanadium
e) Titanium
f) Sulfor
As far as the contamination in a VRB is concerned, the Aluminium and Titanium have a negative impact on the performance. The other materials how ever are neutral.
Conclusion
The recycling components produced can be manufactured easily and cost-effective and can be integrated without any problems into a redox flow test system. For the SEM as a testing system, the corresponding characteristics can be measured immediately. But only on a small spot. The SEM shows, however, that is very well suited for testing critical and non critical materials. Critical Materials in a VRB are Iron, Aluminium and Titatnium, where as Calcium, Zirconium, Vanadium and Sulfor are uncritical [7]. As a next step it is worth to consider other materials such as the frame or the Membrane in order to decide to use recyclable material.
For more article in Peer Reviewed Material Science Journals please click on below link https://crimsonpublishers.com/rdms/


Comments
Post a Comment